What is pH? pH and CEC Effects of pH on nutrients More Info
Adapted from Terry Bates and Tony Wolf. 2008. Nutrient management. In Tony K. Wolf (Ed.), Wine Grape Production Guide for Eastern North America (pp. 141-168). Ithaca:NRAES.
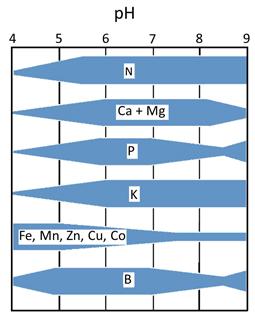
Impact of soil pH on nutrient availability.
What is pH?
Soil pH is a measure of acidity or alkalinity [the activity of hydrogen (H) ions in the soil solution]. The pH scale is numbered from 0 to 14, with value of 7 being neutral. pH values less than 7 reflect acidity, whereas numbers above 7 indicate alkaline conditions. The pH scale is logarithmic (pH = –log H); a pH of 5.0 is ten times more acidic than a pH of 6.0 and one hundred times more acidic than a pH of 7.0.
Soil pH, which can range from 3 to 10, is affected by many factors, including the parent material, the amount of organic matter, the degree of soil leaching by precipitation, and additions of lime or acidifying fertilizers. While soil pH can be precisely quantified, qualitative terms are often used to describe soil pH based on grapevine performance. From a viticultural standpoint, “strongly acidic” soil will generally be a pH of 5.5 or lower and may exhibit mineral-nutrient imbalances. “Slightly acidic” (with a pH of 5.6–6.9) and neutral (with a pH of 7.0) vineyard soils generally have better nutrient balance for plant growth. Calcareous soils—those that contain free calcium carbonate—may be “slightly to strongly alkaline”, with soil pH greater than 7.0 or greater than 8.5, respectively. Vineyard soils with a pH greater than 7.5 are rare in eastern North America but will typically exhibit nutrient imbalances where they do exist.
pH and CEC
Soil pH, cation exchange capacity (CEC), and base saturation values are used together to determine a lime recommendation (the amount of calcium carbonate equivalent needed to raise the soil pH by a desired amount). First, CEC is directly related to soil’s buffer capacity. It takes more lime to raise a soil pH from 4.5 to 6.0 in a soil with high CEC (that is, a clayey soil with a high percentage of organic matter) than it does in a soil with low CEC (that is, a sandy soil with a low percentage of organic matter). Second, there is a positive correlation between soil pH and base saturation, but the relationship is not linear. The chemistry of aluminum and carbonate buffer the soil at low and high pH, respectively. Therefore, it takes more lime to change the pH of soils containing aluminum or carbonate than it does to change the soil pH where aluminum and carbonate are not factors.
Effects of pH on nutrients
Aside from improving the percent base saturation, there are other good reasons to maintain soil pH at optimal levels. In strongly acidic soils, high amounts of free aluminum and iron precipitate phosphorus (P) out of the soil solution, making P unavailable to the plant. Aluminum toxicity can also affect root growth by inhibiting cell division in the root apical meristem. In addition, soil microorganisms, which improve soil fertility through the breakdown of organic matter and metabolism of nitrogen, can be inhibited in acidic soils.
The availability of micronutrients (for example, zinc, iron, manganese, and copper) also changes with soil pH. In general, availability is high in acidic soils and low in alkaline soils. High availability at low soil pH can cause direct toxicity symptoms on the vine or cause indirect deficiency of another element. For example, high availability of zinc and iron may limit phosphorus availability for root uptake. In contrast, low availability of zinc and iron at high soil pH can lead to zinc or iron deficiency. Zinc deficiency is common in California vineyards with sandy, high pH soils. Some grape varieties are susceptible to iron deficiency in vineyards where the soil is alkaline, cool, and wet.
Recommended Resources
Wine Grape Production Guide for Eastern North America. ed. Tony K. Wolf. NRAES, Ithaca. Available for $75.
Monitoring Grapevine Nutrition
Raising Soil pH and Soil Acidification
Essential Nutrients and their Roles
Soil pH and Nutrient Availability
Reviewed by Eric T. Stafne, Mississippi State University, and Bruce Bordelon, Purdue University
